Take high quality sand, soda ash, dolomite, limestone, saltcake and broken glass (cullet) and melt at white heat to a highly viscous consistency. Let the mixture digest for a time - and you are well on the way to making one of the world's most important materials. This is the basic recipe for float glass, one of the greatest of all industrial process inventions, comparable with Bessemer's innovations in steel manufacturing. In fact, this is the formula for many types of mass-produced glass; except that float demands highly exacting standards of quality, care and control beyond those of other everyday uses of glass.
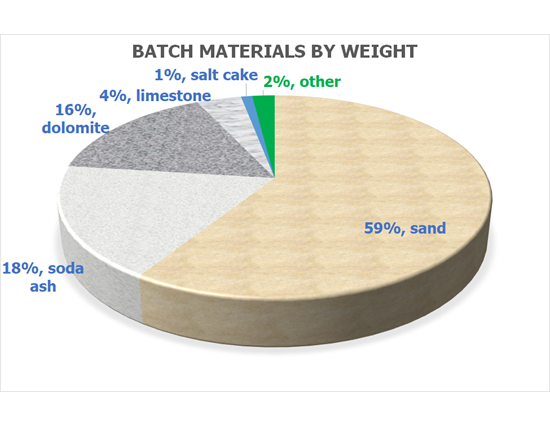
All glass makers like cullet! The more cullet we use, the less virgin raw material is required and the less fuel we need to melt the glass – all of which helps us reduce our environmental impact. Today about 1/3 of our glass comes from cullet and every day we are working to increase this proportion. Whatever the source of raw materials or cullet the composition of float glass is typically:
|
Material
|
% by weight
|
Source
|
|
Silica, SiO2
|
71
|
Sand
|
|
Soda, Na2O
|
14
|
Soda ash, feldspar, salt cake
|
|
Lime, CaO
|
9
|
Dolomite and limestone
|
|
Magnesia, MgO
|
4
|
Dolomite
|
|
Alumina, Al2O3
|
0-3
|
Sand, feldspar
|
|
Others (Iron oxide, colourants etc.)
|
1-3
|
|